NGC 6888 Crescent (Medusa?) Nebula (APOD 13 Aug 2008)
Posted: Wed Aug 13, 2008 4:47 am
APOD and General Astronomy Discussion Forum
https://asterisk.apod.com/




That is the big picture, the main distribution of baryonic matter, the 4% matter known. In ordinary stars H is converted (fused) into He. When stars get hotter during the autumn of their life, they convert He into C and O. Still later towards and within the winter of their life, Si and Fe can be produced, the metals you mention.You wrote: I keep hearing that the "big bang" supposedly left us with primarily hydrogen and helium, and that all other heavier elements ("metals" in star-speak) have been made in stars or supernovae...
The explanation of our APOD masters at todays APOD mentions the release of one solar mass per 10 ky. The glowing matter you see is IMO part of the 'shedding' of the central star.NoelC wrote:The glowing teal-colored oxygen in Tony's image seems to be part of the interstellar medium, glowing possibly as a result of compression, and not part of the material coming off the Wolf-Rayet star. Perhaps I'm wrong here, but if not:
From the wikipedia (http://en.wikipedia.org/wiki/Stellar_evolution)BMAONE23 wrote:Many people have proposed that stars form layers like onions as opposed to a generalized mixing of gasses. Perhaps the Oxygen might be just that particular layer of the star that was previously shed
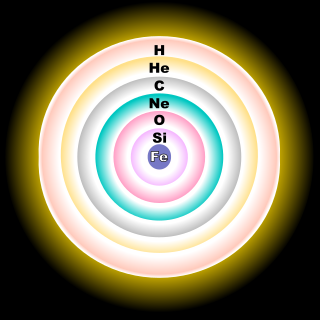
----------------------------------------------henk21cm wrote:From the wikipedia (http://en.wikipedia.org/wiki/Stellar_evolution)BMAONE23 wrote:Many people have proposed that stars form layers like onions as opposed to a generalized mixing of gasses. Perhaps the Oxygen might be just that particular layer of the star that was previously shed
Density and reactivity. Iron is significantly denser than silicon, and so the majority of the iron in Earth's make-up sank to the core of the planet as it was settling down, while the lighter silicates floated to the top. Also, SiO2 is a very stable molecule, compared to iron oxides which become soluble in acidic conditions.henk21cm wrote:Nevertheless your thoughts triggered a philosophical question. On earth, the moon, Venus, Mercury, Mars and the moons of the gas giants Silicon seems to the the abundant element. Si is one of the elements in the nuclear fusion chain of stars. Fe (Iron) is the last one. So there must be somewhat more Si than Fe. Nevertheless the abundancy of Si is overwhelming. Why are our beaches made of silicon oxide and not of grains of rust (iron oxide)?
Iron is dense, and sinks to the center of large objects while they are still molten. This is certainly true for all of the terrestrial bodies you list above. It is the outer portions of large bodies that is rich in Si, a light element. And in the case of Mars, there is still enough iron oxide on its "beaches" to make it the Red Planet.henk21cm wrote:Nevertheless your thoughts triggered a philosophical question. On earth, the moon, Venus, Mercury, Mars and the moons of the gas giants Silicon seems to the the abundant element. Si is one of the elements in the nuclear fusion chain of stars. Fe (Iron) is the last one. So there must be somewhat more Si than Fe. Nevertheless the abundancy of Si is overwhelming. Why are our beaches made of silicon oxide and not of grains of rust (iron oxide)?
Altough it is Chris' quote, Qev's answer is similar. Basically it is the well known fact that an object with a volumetric mass larger than that of water does not float on water. I was aware of that idea, however it does not fully account for the natural occurence of iron.Chris Peterson and Qev wrote: Iron is dense, and sinks to the center of large objects while they are still molten. This is certainly true for all of the terrestrial bodies you list above. It is the outer portions of large bodies that is rich in Si, a light element. And in the case of Mars, there is still enough iron oxide on its "beaches" to make it the Red Planet.
Surface iron is rare, in comparison to the core, which is where nearly all of it ended up. The key point- which you make- is that we find iron ore. We don't find veins of metallic iron (they do exist, but are extremely rare). We find iron bound up with other elements, in complex minerals.henk21cm wrote:The ρ of iron is nearly 8000 kg/m³, the ρ of silicon is nearly 2500 kg/m³. Since a lot of time has gone since the earth was liquid, there was an ample amount of time for iron to sink to the center of the earth, whereas silicon would rise to the outer range of the earth. As a result iron would be a rare commodity. To find iron or iron ore, one should have to dig rather deep, at least thousands of kilometers. Since volcanoes are outlets of the inner earth, the material spewn out has an origin usually not very deep, of the order of 10 km. That can not account for the not so sporadic availability of iron.
As stated in the Wikipedia entry, this illustration is only for the core of a massive star having at least 8 solar masses. For a less massive star, like the Sun, the fusion reaction would stop at the carbon stage, resulting in a carbon-oxygen white dwarf.henk21cm wrote:From the wikipedia (http://en.wikipedia.org/wiki/Stellar_evolution)BMAONE23 wrote:Many people have proposed that stars form layers like onions as opposed to a generalized mixing of gasses. Perhaps the Oxygen might be just that particular layer of the star that was previously shed
I would have thought that since iron is the very last fusion product in a massive star's core, it would all be squeezed into neutrons (proton + electron -> neutron + neutrino) during the core collapse, resulting in either a neutron star or, if the core collapse doesn't stop there, a black hole. So, none of the iron would have escaped from the core in a supernova blast.henk21cm wrote: Nevertheless your thoughts triggered a philosophical question. On earth, the moon, Venus, Mercury, Mars and the moons of the gas giants Silicon seems to the the abundant element. Si is one of the elements in the nuclear fusion chain of stars. Fe (Iron) is the last one. So there must be somewhat more Si than Fe. Nevertheless the abundancy of Si is overwhelming. Why are our beaches made of silicon oxide and not of grains of rust (iron oxide)?
At elementary school the Kiruna mines in Sweden were taught to contain rather pure iron. I do remember about 70%, Google learned me that during nearly 50 years my memory slipped a little, the ore found it is just 60% pure. Later during chemistry class we learned the process to create iron from ore: use cokes and hydrogen gas to bind the oxygen to the hydrogen. Browsing the handbook of chemistry and physics i foud that iron oxyde melts at about 1500 C, silicium oxyde at about 1700 C. So there is not much difference between the two melting points. The density of iron oxide is about 5500 kg/m³, the density of silicium oxide is 2200 kg/m³. Iron oxide will sink just like pure iron to the core, whereas the silicium oxide will float on top.Chris Peterson wrote: Surface iron is rare, in comparison to the core, which is where nearly all of it ended up. The key point- which you make- is that we find iron ore. We don't find veins of metallic iron (they do exist, but are extremely rare). We find iron bound up with other elements, in complex minerals.
The Earth didn't end up as an onion with neat shells of decreasing density for many reasons: it cooled fairly quickly, there was mixing- turbulent and not, elements reacted to form compounds, local cooling and crystallization created traps that stopped differentiation.
starnut wrote:As stated in the Wikipedia entry, this illustration is only for the core of a massive star having at least 8 solar masses. For a less massive star, like the Sun, the fusion reaction would stop at the carbon stage, resulting in a carbon-oxygen white dwarf.
Be cautious how you interpret melting points. These are normally given for standard atmospheric pressure. At the extreme pressures found deep in the Earth, material properties are very different, and not always well understood. Even at very high temperatures, materials are extremely viscous. The rate at which a denser material will fall through a less dense one is extremely slow, and convective effects may dominate.henk21cm wrote:Browsing the handbook of chemistry and physics i foud that iron oxyde melts at about 1500 C, silicium oxyde at about 1700 C. So there is not much difference between the two melting points. The density of iron oxide is about 5500 kg/m³, the density of silicium oxide is 2200 kg/m³. Iron oxide will sink just like pure iron to the core, whereas the silicium oxide will float on top.
Certainly, convective processes in the mantle are responsible in part for such mixing. They have nothing to do with the Earth's magnetic field, however. That is produced by electric currents in the outer (liquid) core, which is mostly iron, and doesn't interact strongly with the overlying mantle.Between the earth' crust and the inner core massive convection zones exist. The movement hot material is responsible for the earth' magnetic field. (It can't be any ferromagnetic effect of the iron core, since its temperature is far above the Curie temperature, of about 1000 K.) These convection zones might be responsible for some mixing of silicium- and iron ores.