Page 1 of 1
Properties of sulfuric acid (APOD 26 Feb 2008)
Posted: Tue Feb 26, 2008 10:08 am
by tnzkka
The text going with today's APOD states: "...recombined into the volatile sulfuric acid."
Wikipedia states about sulfuric acid: Melting point 283 K (10 degrees C); Boiling point 610 K (337 degrees C)
Substances with boiling- and meltingproperties like these do not seem to be volatile to me.
How comes sulfuric acid is calles volatile in this text?
Posted: Tue Feb 26, 2008 11:43 am
by Czerno
> How comes sulfuric acid is calles volatile in this text?
I guess it's taking account of the temperatures & pressions in Venus's atmosphere...
Posted: Tue Feb 26, 2008 12:57 pm
by tnzkka
I guess it's taking account of the temperatures & pressions in Venus's atmosphere...
Volatylity is a relative notion, I'm aware of that; so OK for ocurring temperature, but nót for occuring pressure (on ground level of course) therefore I'm asking here.
Posted: Tue Feb 26, 2008 1:02 pm
by NoelC
Perhaps not the scientific meaning but more the general one ("Volatility, a measure of instability"). I read it as more along the lines of "recombined into the nasty sulfuric acid". Stuff that's likely to combine with you and eat you up.
I sure wouldn't want to be there under a rain of that stuff.
I wonder what the chances are that Venus could ever clear off and be a nice place to visit. Probably pretty slim, considering it's still pretty volatile after 5 billion years...
-Noel
Posted: Tue Feb 26, 2008 1:21 pm
by Dr. Skeptic
Seed the atmosphere with the right bacteria then sit back an wait a few million years - see what happens.
Posted: Tue Feb 26, 2008 2:03 pm
by emc
Dr. Skeptic wrote:Seed the atmosphere with the right bacteria then sit back an wait a few million years - see what happens.
Space and time are too restrictive... It is clear we will eventually need somewhere else to planet

or else change our habits due to the habitat destruction/construction and afternoon rush hour monsters that continue to grow.
Posted: Tue Feb 26, 2008 6:25 pm
by Arramon
Love that image of venus... the flaming torch of the heavens.
But, I'd rather we move away from the sun than towards it. We really need to be concentrating on the moons that are in this solar system. So many out there, we know so little, and yet we know that they contain so many resources that make the resources here on earth seem like a small teaspoon in comparison.
Do you want to sweat it out and remove more clothes to fight the summer heat or do you want to throw on more clothes to fight the chill of winter? You can add as many clothes/warming items as you'd like, but you can only take off so many garments or use so many resources to keep cool when the temperature is off the hook (such as venus is).
Mysterious Acid Haze on Venus
Posted: Tue Feb 26, 2008 8:35 pm
by ajhill
The source of the acid clouds wouldn't seem to much of a mystery. Isn't the most likely cause vulcanic eruptions?
Posted: Tue Feb 26, 2008 8:46 pm
by NoelC
Arramon wrote:Do you want to sweat it out and remove more clothes to fight the summer heat or do you want to throw on more clothes to fight the chill of winter?
Personally, I am no fan of heat and so I might put my faith in air conditioning (solar powered, of course). But I think it might be ultimately more possible to colonize a planet that has an atmosphere (i.e., opposed to Mars).
Now, Europa... Some of the others... Very intriguing. Energy might prove to be a bit scarce, though.
-Noel
Posted: Tue Feb 26, 2008 11:25 pm
by Arramon
NoelC wrote:
Now, Europa... Some of the others... Very intriguing. Energy might prove to be a bit scarce, though.
-Noel
Unless we become so technologically advanced that we can siphon the energy already created by the magnetic fields of saturn and its moons. =)
There is so much energy out there.. even far far from earth, that we should never run out. So many resources to collect and refine, not just solar. I think once we get out in open space, outside of earth's pull, and colonize/harvest the moon, we can then work towards moving outward from there.
And if we ever run out of acidic substances, we can just go back to venus and gather some. =b
Could the atmosphere of venus be volatile in any way that something might be ignited if a certain reaction were to be caused from the mixture of just the right components? Boom! There goes half of that world.
Like, we wouldn't wanna go there if it turns into a giant lump of C4 or something crazy, and our little satellite's, unknowingly, set off an electrical charge that detonates the combined mixtures in the atmosphere.
O.o
*eats the skin of venus like its a lemon peel*
mmmmm.... acidic.
Posted: Wed Feb 27, 2008 12:16 am
by Case
Arramon wrote:Could the atmosphere of venus be volatile in any way that something might be ignited if a certain reaction were to be caused from the mixture of just the right components? Boom! There goes half of that world.
Like, we wouldn't wanna go there if it turns into a giant lump of C4 or something crazy, and our little satellite's, unknowingly, set off an electrical charge that detonates the combined mixtures in the atmosphere.
There are
indications that natural lightning occurs on Venus, so we shouldn't be too worried about a little spark we could set by going there.
Posted: Wed Feb 27, 2008 1:15 am
by rigelan
And for most reactions, you need TWO unstable components. Most of why explosions exist on earth is because of some hydrocarbon react with the oxygen (20% of atmosphere). If there were two naturally explosive compounds near each other, I'm sure the lightning would take care of them rather quickly.
Wandering away from the initial question
Posted: Wed Feb 27, 2008 12:00 pm
by tnzkka
Please, ladies and gentlemen,
Will you please realise how few of the "answers/replies" above relate to my initial question and how many "answers/replies" are just wandering away?
To quote myself:"How comes sulfuric acid is called volatile in this text?" (Unquote myself)
Thank you for cooperating.
TNZKKA, topic-starter.
Re: Wandering away from the initial question
Posted: Wed Feb 27, 2008 12:22 pm
by emc
tnzkka wrote:Please, ladies and gentlemen,
Will you please realise how few of the "answers/replies" above relate to my initial question and how many "answers/replies" are just wandering away?
To quote myself:"How comes sulfuric acid is called volatile in this text?" (Unquote myself)
Thank you for cooperating.
TNZKKA, topic-starter.
I am not sure, but isn't sulfuric acid volatile when mixed with water?... doesn't the mixture heat up??? Perhaps some of the other elements on Venus provide a medium for a more violent reaction? Sorry to be so vague... just wanted to try and help.
Re: Wandering away from the initial question
Posted: Wed Feb 27, 2008 2:11 pm
by Dr. Skeptic
emc wrote:tnzkka wrote:Please, ladies and gentlemen,
Will you please realise how few of the "answers/replies" above relate to my initial question and how many "answers/replies" are just wandering away?
To quote myself:"How comes sulfuric acid is called volatile in this text?" (Unquote myself)
Thank you for cooperating.
TNZKKA, topic-starter.
I am not sure, but isn't sulfuric acid volatile when mixed with water?... doesn't the mixture heat up??? Perhaps some of the other elements on Venus provide a medium for a more violent reaction? Sorry to be so vague... just wanted to try and help.
H2SO4 is not volatile when mixed with H2O, it becomes a weaker solution. Caution is needed when mixing H2SO4 and H2O, if the H2O has any impurity dissolved or in suspension, the impurities can react violently setting up a very undesirable situation.
H2SO4 is Volatile be cause it has a very high reactive quotient, it can violently react with a large number of elements and compounds -especially with the organic molecules of your flesh and most anything metallic.
Because Venus doesn't have a magnetic field terraforming Venus is most likely not practical.
Re: Properties of sulfuric acid (APOD 26 Feb 2008)
Posted: Wed Feb 27, 2008 2:31 pm
by bystander
tnzkka wrote:How comes sulfuric acid is calles volatile in this text?
With the surface temperature of
Venus at 460 C and the boiling point of
sufuric acid at 290 C, I imagine it evaporates quite easily, which is the primary meaning of
volatile.
Re: Properties of sulfuric acid (APOD 26 Feb 2008)
Posted: Wed Feb 27, 2008 6:07 pm
by Arramon
bystander wrote:tnzkka wrote:How comes sulfuric acid is calles volatile in this text?
With the surface temperature of
Venus at 460 C and the boiling point of
sufuric acid at 290 C, I imagine it evaporates quite easily, which is the primary meaning of
volatile.
d'oh! the answer to the first post comes on page 2...????? =b
I know...at last!!
Posted: Wed Feb 27, 2008 7:09 pm
by tnzkka
Thank you,
bystander,
I should not only have looked up the properties of sulfuric acid, but of Venus' surface too.
Taught me a good lesson for next time!

Bye.
Posted: Wed Feb 27, 2008 7:31 pm
by bystander
Arramon wrote:d'oh! the answer to the first post comes on page 2...
Actually, Czerno had the answer with the second post.
Czerno wrote:I guess it's taking account of the temperatures & pressions in Venus's atmosphere...
However, with the primary atmospheric gas on Venus being carbon dioxide and the probable presence of hydrocarbons, I'm wondering if maybe Dr. Skeptic had the definitive answer. (secondary definition - explosive).
Dr. Skeptic wrote:...Caution is needed when mixing H2SO4 and H2O, if the H2O has any impurity dissolved or in suspension, the impurities can react violently setting up a very undesirable situation.
H2SO4 is Volatile be cause it has a very high reactive quotient, it can violently react with a large number of elements and compounds -especially with the organic molecules of your flesh and most anything metallic...
But I'm relatively sure that the first definition is implied, since the APOD is of clouds of sulphuric acid.
Mysterious Acid Haze on Venus (APOD 2008 Feb 26)
Posted: Thu Feb 28, 2008 2:28 am
by ne0357
After years of studying chemistry, the first time I read of sulfuric acid’s volatility was from a short story by Edgar Allen Poe, “The Thousand-and-Second Tale of Scheherazade”:
Place in a platina crucible over a spirit lamp, and keep it a red heat; pour in some sulphuric acid, which though the most volatile of bodies at a common temperature, will be found to become completely fixed in a hot crucible, and not a drop evaporates—being surrounded by an atmosphere of its own, and does not, in fact, touch the side. A few drops of water are now introduced, when the acid, immediately coming in contact with the heated sides of the crucible, flies off in sulphurous acid vapor, and so rapid is its progress, that the caloric of the water passes off with it, which falls a lump of ice to the bottom; by taking advantage of the moment before it is allowed to re-melt, it may be turned out a lump of ice from a red-hot vessel.
All of this got me thinking. Have astrobiologists considered the possibility of sulfur-based or sulfuric acid based life on Venus? There are obviously rich complex energy cycles involving SO2, SO3, water and sulfuric acid. The idea of an organism ingesting water and expiring sulfuric acid might be a direct analogy to us inhaling O2 and exhaling CO2. Complex sulfur chemistry is not particularly well studied on this planet, and sulfur compounds as we understand them can’t hold ‘chemical information’ the same way carbon compounds can, so it’s really hard to dream up what it might look like. Hard to even guess what you’d look for…
Sulphuric Acid Clouds On Venus
Posted: Thu Feb 28, 2008 12:46 pm
by videograham
If you look at the lowest cloud in the picture (26th Feb), the left-hand side seems to taper outwards from a point.
Is it possible that the cloud's origins are volanic?
Could the products of an eruption reacted with material in the atmosphere to produce the acid cloud?
The spreading outwards of the cloud from this point is consistent with pictures of volcanoes, chimneys, fires etc taken from space.
Posted: Thu Feb 28, 2008 2:09 pm
by Dr. Skeptic
Posted: Thu Feb 28, 2008 9:04 pm
by Arramon
Here are other images taken of the cloud structure on Venus throughout July 2007.
The southern region has a very prominent vortex of some sort. Other moons in the solar system do have atmospheres that are created by the plumes of erupting mounds... maybe this is similar. Or possibly, in the southern region, there is a mixture occuring at a certain location that is pumping out the acidic clouds. It doesn't look like its a global haze on the planet, but comes from the southern region and wraps around Venus.
Anyone explanations?
July 8, 2007

July 23, 2007
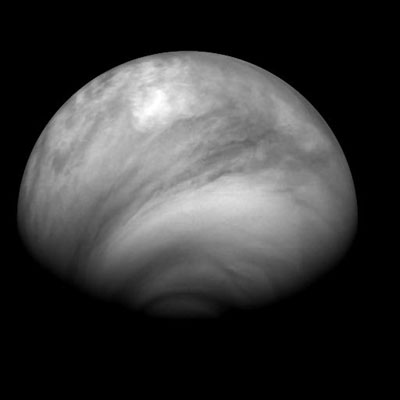
July 24, 2007

July 27, 2007
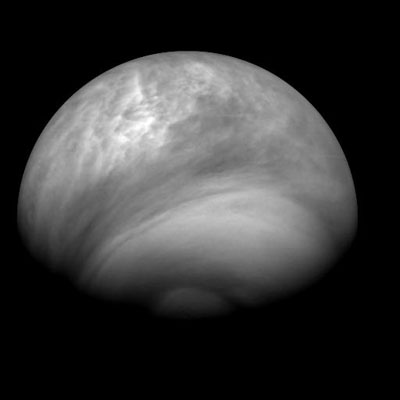
July 28, 2007

July 30, 2007

August 2, 2007

Molecular structure of Venus Atmosphere
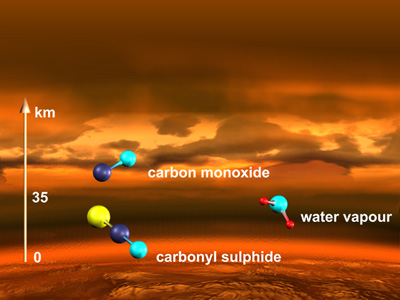
*images from ESA website
http://www.esa.int/SPECIALS/Venus_Express/index.html
Posted: Fri Feb 29, 2008 12:22 am
by Arramon
New article concerning Venus being made from a collision with another forming blob of meh....
http://www.space.com/scienceastronomy/0 ... ision.html







