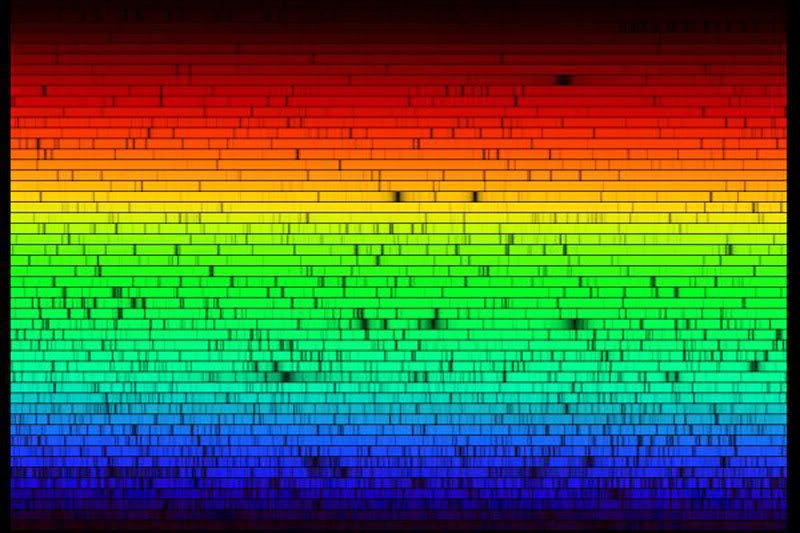
All the colors of the sun (APOD 24 June 2007)
All the colors of the sun (APOD 24 June 2007)
Just wondering if the sun spectrum has ever been compared to that of a nuclear explosion. If a spectrum hasn't been done in the past, it's too late now, but I wonder if the "missing" colors of the sun might be missing from a nuclear blast also. Just a thought.
Take care,
Chip
Chip
Interestingly though the spectrum shown
http://antwrp.gsfc.nasa.gov/apod/ap070624.html
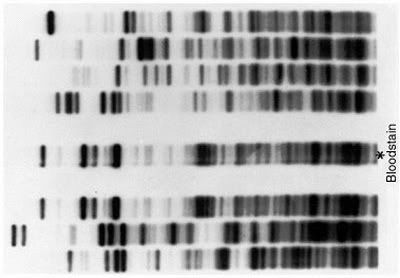
resembles this
http://images.ask.com/fr?q=dna+fingerpr ... 253D1&qt=0
a DNA fingerprint. Could it be a spectral fingerprint???
http://antwrp.gsfc.nasa.gov/apod/ap070624.html
resembles this
http://images.ask.com/fr?q=dna+fingerpr ... 253D1&qt=0
a DNA fingerprint. Could it be a spectral fingerprint???
It is still not known why the Sun's light is missing some co
The Sun does not emit white light.
-
TimeTravel123456789
- Ensign
- Posts: 25
- Joined: Mon Dec 04, 2006 1:52 am
- Contact:
Atmospheric effects
My question is about the amount of atmospheric effect in that spectral image. I am pretty sure that observatory is land based. How much of the image has atmospheric chemicals visible?
There are still tests of nuclear weapons. The Comprehensive Test Ban Treaty, I believe, was not signed by the United States.
You could always email the Department of Defense to ask if they have a spectrum. They would probably say yes. They could also take one. There are weapons tests I am pretty sure.
The spectrum would be different. I think most of our nuclear devices are not based on HH HE reactions. They are more based on Ur and other fission reactions. I do not believe we use fusion bombs.
General Electric does play a role I believe in nuclear development; I am pretty sure a certain class of submarine is influenced by them. They could also help you with your question.
The DNA issue is cool. I did not want to click so as to avoid spyware. Sad that I limit interaction to avoid being preyed upon. Too paranoid...yes sometimes.
Have a good day.
There are still tests of nuclear weapons. The Comprehensive Test Ban Treaty, I believe, was not signed by the United States.
You could always email the Department of Defense to ask if they have a spectrum. They would probably say yes. They could also take one. There are weapons tests I am pretty sure.
The spectrum would be different. I think most of our nuclear devices are not based on HH HE reactions. They are more based on Ur and other fission reactions. I do not believe we use fusion bombs.
General Electric does play a role I believe in nuclear development; I am pretty sure a certain class of submarine is influenced by them. They could also help you with your question.
The DNA issue is cool. I did not want to click so as to avoid spyware. Sad that I limit interaction to avoid being preyed upon. Too paranoid...yes sometimes.
Have a good day.
James T. Struck
- Pete
- Science Officer
- Posts: 145
- Joined: Sun Jan 01, 2006 8:46 pm
- AKA: Long John LeBone
- Location: Toronto, ON
The Sun's spectrum is very, very different from that of the energy-producing
reactions taking place in the Sun's interior: pp fusion produces hard gamma
rays, which, over millions of years, are attenuated to lower energies as they
make their way to the surface. The solar spectrum ends up being very close
to that of a 5730 K blackbody emitter. If you built a chemically identical
replica of the Sun's surface (from the photosphere outward) and heated up
each layer to the temperature of its solar counterpart through non-nuclear
means, the replica's spectrum would be indistinguishable from the Sun's.
TimeTravel, I wrecklessly clicked on BMAONE23's link, which didn't cause my
computer to profess its love for me and subsequently explode. That's a
harmless link in my books! Here's a shorter link to the same image anyway:
http://www.mun.ca/biology/scarr/F14-03_ ... rprint.jpg
EDIT: manual line breaks. *shakes fist at hugeass link*
reactions taking place in the Sun's interior: pp fusion produces hard gamma
rays, which, over millions of years, are attenuated to lower energies as they
make their way to the surface. The solar spectrum ends up being very close
to that of a 5730 K blackbody emitter. If you built a chemically identical
replica of the Sun's surface (from the photosphere outward) and heated up
each layer to the temperature of its solar counterpart through non-nuclear
means, the replica's spectrum would be indistinguishable from the Sun's.
TimeTravel, I wrecklessly clicked on BMAONE23's link, which didn't cause my
computer to profess its love for me and subsequently explode. That's a
harmless link in my books! Here's a shorter link to the same image anyway:
http://www.mun.ca/biology/scarr/F14-03_ ... rprint.jpg
EDIT: manual line breaks. *shakes fist at hugeass link*
-
jimmysnyder
- Ensign
- Posts: 68
- Joined: Tue Jun 12, 2007 2:32 pm
- Location: New Jersey, USA
- Contact:
What you are seeing in the solar spectrum is light of virtually all frequencies except those subtracted by atoms in the solar atmosphere. If you looked at the spectum of a nuclear explosion, you would see the light of the explosion with the missing frequencies subtracted by atoms in our atmosphere. So that aspect the spectra would be incommensurate.
It was noted in the APOD that sunlight appears brightest in yellow-green. Perhaps the light of a thermonuclear explosion would be the same. However, the explosion is triggered by a fusion explosion. That might emit a preponderance of light in some other frequency range.
I expect that spectra of thermonuclear explosions have been recorded, but I don't know how to get an image of one.
It was noted in the APOD that sunlight appears brightest in yellow-green. Perhaps the light of a thermonuclear explosion would be the same. However, the explosion is triggered by a fusion explosion. That might emit a preponderance of light in some other frequency range.
I expect that spectra of thermonuclear explosions have been recorded, but I don't know how to get an image of one.
Making mistakes since 1950.
I noticed the dominance of the green bands within the spectrum. Could that possibly have any thing to do the evolution of plant life on this planet?
A. Baca
aabaca001@msn.com
aabaca001@msn.com
Green dominance would probably not directly affect the plant evolution, I believe.
Notice how plants are green?
That is because they reflect green light and absorb the rest. They use Red, Yellow, Cyan and Blue to make their sugar and not the green color.
Although it is a good thing that our eyes can see the colors they do if there is so much red green and blue in our light. Coincidence? Maybe.
Notice how plants are green?
That is because they reflect green light and absorb the rest. They use Red, Yellow, Cyan and Blue to make their sugar and not the green color.
Although it is a good thing that our eyes can see the colors they do if there is so much red green and blue in our light. Coincidence? Maybe.
but what about the 'missing' light?
Does anyone here know the radius of the sun in light-seconds? Likewise, the volume of the area of the sun where fusion occurs? I am trying to imagine just how slow gamma rays, and their offspring, can be made to go.
Also, might someone tell me if the spectra of other stars also have 'missing' lines of emission? How likely, or unlikely, is it that each star is unique in it's spectral emission?
Also, might someone tell me if the spectra of other stars also have 'missing' lines of emission? How likely, or unlikely, is it that each star is unique in it's spectral emission?
Re: but what about the 'missing' light?
The Sun is roughly 2.3 light-seconds in radius, or 700 million meters. The core of the sun (where fusion reactions occur) is thought to be about one fifth of this radius, 0.46 light-seconds or ~140 million meters. Estimates for the time taken for energy to escape the core to be emitted as light from the surface range from 17000 to 50 million years; the mean path length for a photon in the Sun is extremely short.jimsaruff wrote:Does anyone here know the radius of the sun in light-seconds? Likewise, the volume of the area of the sun where fusion occurs? I am trying to imagine just how slow gamma rays, and their offspring, can be made to go.
Also, might someone tell me if the spectra of other stars also have 'missing' lines of emission? How likely, or unlikely, is it that each star is unique in it's spectral emission?
As far as I know, all stars show some absorption spectra; the lines they show depend on makeup and temperature.
http://en.wikipedia.org/wiki/Stellar_classification
Don't just stand there, get that other dog!
Well, our eyes likely evolved to take advantage of the most abundant frequencies of light from the Sun, which fall in the yellow-green part of the spectrum. The human eye is most sensitive to yellow light, apparently.rigelan wrote:Green dominance would probably not directly affect the plant evolution, I believe.
Notice how plants are green?
That is because they reflect green light and absorb the rest. They use Red, Yellow, Cyan and Blue to make their sugar and not the green color.
Although it is a good thing that our eyes can see the colors they do if there is so much red green and blue in our light. Coincidence? Maybe.
It's been theorized that, early on in Earth's history, the dominant photosynthetic organisms were taking advantage of the abundance of green light from the Sun... which means the photosynthetic organisms would have looked purple to our eyes. Be interesting if they'd have hung around, we'd have purple trees.
Green pigment photosynthesis (using the red-blue parts of the spectrum) would then have evolved to take advantage of the 'leftovers' not being used by the dominant photosynthetic organisms (life likes to fill every niche it can!). Then at some point, something happened to cause these dominant species to vanish, leaving behind the green chlorophyll-based organisms as the primary photosynthetic organisms.
It's a pretty controversial theory at this point, but it's interesting. I'd have to wonder why green light photosynthesis wouldn't re-evolve, though...
Don't just stand there, get that other dog!
-
jimmysnyder
- Ensign
- Posts: 68
- Joined: Tue Jun 12, 2007 2:32 pm
- Location: New Jersey, USA
- Contact:
Re: but what about the 'missing' light?
Yes, when cosmologists speak of 'red shift' they are talking about the shift of the missing lines toward the red.jimsaruff wrote:Also, might someone tell me if the spectra of other stars also have 'missing' lines of emission?
Making mistakes since 1950.
Well, to answer that, I couldn't find better words than those thrown at me once at other thread:BMAONE23 wrote:OK OK OK MAKC
see my revision above, If you want colorful, I'll give that too.
[edited image]
[u]nomore[/u] wrote:Why don't you just add a bee to the image?
That would bee just as scientific as this approach.
Yup! Gamma rays are the highest-energy domain of electromagnetic radiation (photons), although there is some overlap between 'high-energy x-rays' and 'low-energy gamma rays'; it's not really a hard-set boundary. I think gamma rays are considered to begin someplace above a frequency of 30,000 PHz (petahertz, 10^15 Hz).jimsaruff wrote:Thanks, Qev, for your snappy response. I thought I might do a quick read of your link to wiki...hahaha...and decided to respond this week instead.
So, are gamma rays 'photons' at high frequencies?
Don't just stand there, get that other dog!
Thanks, Jimmy. Are they the same 'missing lines' as the sun's?
Qev, how short can radiation get and still be considered a 'ray'? Or, perhaps more clearly, how short can a ray get and still be a 'wave' or have amplitude?
What's bugging me is how dense can the sun be that it might take 50 million years for EM energy to get 'out'. Doesn't slowing EM down to turnstyle speed turn it into pure infrared?
How do the UV rays get into sunshine anyway?
Thanks for sharing your knowledge.
Qev, how short can radiation get and still be considered a 'ray'? Or, perhaps more clearly, how short can a ray get and still be a 'wave' or have amplitude?
What's bugging me is how dense can the sun be that it might take 50 million years for EM energy to get 'out'. Doesn't slowing EM down to turnstyle speed turn it into pure infrared?
How do the UV rays get into sunshine anyway?
Thanks for sharing your knowledge.
As far as I know, a light ray or wave can have any amplitude and frequency. The problem is that if we get an amplitude too large or small, we have no equipment to measure it anymore. Same is true with the frequency.
Any light wave can also be called a light ray.
Gamma rays refer to a specific group of high frequency light waves just like visible light, microwaves, and radio waves are also light rays.
Any light wave can also be called a light ray.
Gamma rays refer to a specific group of high frequency light waves just like visible light, microwaves, and radio waves are also light rays.
Infrared refers to a specific frequency of light, not the speed of the light.Doesn't slowing EM down to turnstyle speed turn it into pure infrared?
-
jimmysnyder
- Ensign
- Posts: 68
- Joined: Tue Jun 12, 2007 2:32 pm
- Location: New Jersey, USA
- Contact:
Not exactly, it depends upon what elements are found in the atmosphere of the star. In order to make this explanation simpler, I will say that a line is present rather than say that anything is missing. Each element has its own particular set of lines. If a star has a certain element, then the attendant lines will be present. Most, if not all, stars have hydrogen so those lines are almost always present.jimsaruff wrote:Are they the same 'missing lines' as the sun's?
Of course the light travels at the speed of light, no slower, no faster. However it careens around inside the star like a pinball. On average, it takes quite some time to escape to the surface. Since all light travels at the same speed, the color of light cannot depend on the speed. Instead it depends upon the energy of the light.jimsaruff wrote:What's bugging me is how dense can the sun be that it might take 50 million years for EM energy to get 'out'. Doesn't slowing EM down to turnstyle speed turn it into pure infrared?
UV is just a range of energies like any other. It is more energetic than visible light, but less than x-rays.jimsaruff wrote:How do the UV rays get into sunshine anyway?
Making mistakes since 1950.
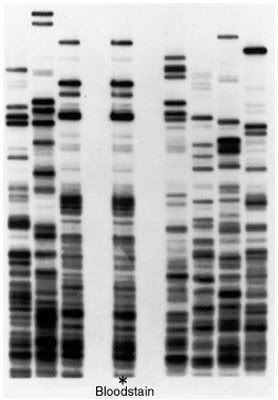 vs
vs