
NGC 6888 Crescent (Medusa?) Nebula (APOD 13 Aug 2008)
- neufer
- Vacationer at Tralfamadore
- Posts: 18805
- Joined: Mon Jan 21, 2008 1:57 pm
- Location: Alexandria, Virginia
NGC 6888 Crescent (Medusa?) Nebula (APOD 13 Aug 2008)
Last edited by neufer on Wed Aug 13, 2008 1:00 pm, edited 1 time in total.
Art Neuendorffer
Re: The Medusa Nebula? (APOD 10 Aug 2008)
Art Neuendorffer has showed a jelly fish, named medusa. There is yet another medusa:
She is a Gorgon daughter. If any mortal looked her in the eys, according to ancient Greek mythology, it changed into a stone statue. Perseus cut off hear head, using his shining shield as a mirror, avoiding looking her in the eyes directly. He liberated the daughter of King Cepheus and queen Cassiopeia, named Andromeda, from a sea monster named Cetus. Cassiopeia had offended one of the Nereids claimning that she was more beautiful than the Nereids. Poseidon sent a seamonster, the Cetus. Andromeda was to be offered to the monster, to ease the Nereids. Perseus showed Medusas head to the monster, which immediatedly became a huge rock. After some remaining fights with previous lovers of Andromeda -just like a B rated action movie- the saga ends.



She is a Gorgon daughter. If any mortal looked her in the eys, according to ancient Greek mythology, it changed into a stone statue. Perseus cut off hear head, using his shining shield as a mirror, avoiding looking her in the eyes directly. He liberated the daughter of King Cepheus and queen Cassiopeia, named Andromeda, from a sea monster named Cetus. Cassiopeia had offended one of the Nereids claimning that she was more beautiful than the Nereids. Poseidon sent a seamonster, the Cetus. Andromeda was to be offered to the monster, to ease the Nereids. Perseus showed Medusas head to the monster, which immediatedly became a huge rock. After some remaining fights with previous lovers of Andromeda -just like a B rated action movie- the saga ends.



Regards,
Henk
21 cm: the universal wavelength of hydrogen
Henk
21 cm: the universal wavelength of hydrogen
Re: The Medusa Nebula? (APOD 10 Aug 2008)
If one looked at Eta Carinae, straight into one of the lobes, would that resemble the Crescent Nebula? How similar are they?


- NoelC
- Creepy Spock
- Posts: 876
- Joined: Sun Nov 20, 2005 2:30 am
- Location: South Florida, USA; I just work in (cyber)space
- Contact:
NGC 6888: The Crescent Nebula - APOD 13 Aug 2008
http://antwrp.gsfc.nasa.gov/apod/ap080813.html
A beautiful job of image processing by Tony Hallas to bring us a visualizaiton of the gaseous parts without being overwhelmed by the bright stars! Bravo Tony!
This unprecedented view of the oxygen gas brings to mind a question, though...
I keep hearing that the "big bang" supposedly left us with primarily hydrogen and helium, and that all other heavier elements ("metals" in star-speak) have been made in stars or supernovae...
The glowing teal-colored oxygen in Tony's image seems to be part of the interstellar medium, glowing possibly as a result of compression, and not part of the material coming off the Wolf-Rayet star. Perhaps I'm wrong here, but if not:
A beautiful job of image processing by Tony Hallas to bring us a visualizaiton of the gaseous parts without being overwhelmed by the bright stars! Bravo Tony!
This unprecedented view of the oxygen gas brings to mind a question, though...
I keep hearing that the "big bang" supposedly left us with primarily hydrogen and helium, and that all other heavier elements ("metals" in star-speak) have been made in stars or supernovae...
The glowing teal-colored oxygen in Tony's image seems to be part of the interstellar medium, glowing possibly as a result of compression, and not part of the material coming off the Wolf-Rayet star. Perhaps I'm wrong here, but if not:
- Could there really be so much oxygen gas just floating around out there? It seems spread pretty evenly through interstellar space in this image.
- Would this imply a rich history of long-dead stars? If so, how did the gas get spread around so evenly? Or does it require a rethink of the "big bang left us with just hydrogen and helium" supposition?
- If it's dead star stuff, does this history mesh with the 13+ billion year theorized life of the universe so far?
Re: NGC 6888: The Crescent Nebula - APOD 13 Aug 2008
G'day NoelC,
Lets assume that what you see is the interstellar gas glowing. The density of interstellar gas is roughly 1 particle per cm³, or 1E6 particles per m³. Further follow our APOD masters: the glowing object is 25 ly in diameter (I assume a sphere). 1 ly is approximately 1E16 m, so the volume of this sphere is of the order of 1E47m³. That means 1E53 particles. 1E24 particles give one mole, so within the sphere there are 1E29 moles of interstellar gas. Lets follow your assumption: the interstellar gas is made of (neutral) oxygen. 1 mole of neutral oxygen has a mass of 0.01 kg, so the mass of the particles within the sphere is 1E27 kg, Jupiter alike mass. The mass of the sun is 2E30 kg. IMO interstellar gas is mainly neutral hydrogen, emitting 21 cm radio waves, but one order of magnitude less in mass. The mass of the insterstellar stuff within the sphere is then 1E26 kg, a mass similar to Neptune.
Now the (mostly mine) speculations are brought into the arena. Lets assume the shedding is taking place for at least 100 years. It must be more than 25 years, otherwise we could not see all parts of the glowing bubble. A mass of 2E28 kg would have been shedded during a period of 100 years. That is one to two orders of magnitude more than the interstaller gas. A logical conslusion is that due to such a difference in density the shedded gas will "outglow" the interstellar gas.
IMHO neither of your three points is the case, since you could re-assess your assumption about what you see in the image: the interstellar gas or the shedded gas.
Nevertheless your thoughts triggered a philosophical question. On earth, the moon, Venus, Mercury, Mars and the moons of the gas giants Silicon seems to the the abundant element. Si is one of the elements in the nuclear fusion chain of stars. Fe (Iron) is the last one. So there must be somewhat more Si than Fe. Nevertheless the abundancy of Si is overwhelming. Why are our beaches made of silicon oxide and not of grains of rust (iron oxide)?
That is the big picture, the main distribution of baryonic matter, the 4% matter known. In ordinary stars H is converted (fused) into He. When stars get hotter during the autumn of their life, they convert He into C and O. Still later towards and within the winter of their life, Si and Fe can be produced, the metals you mention.You wrote: I keep hearing that the "big bang" supposedly left us with primarily hydrogen and helium, and that all other heavier elements ("metals" in star-speak) have been made in stars or supernovae...
The explanation of our APOD masters at todays APOD mentions the release of one solar mass per 10 ky. The glowing matter you see is IMO part of the 'shedding' of the central star.NoelC wrote:The glowing teal-colored oxygen in Tony's image seems to be part of the interstellar medium, glowing possibly as a result of compression, and not part of the material coming off the Wolf-Rayet star. Perhaps I'm wrong here, but if not:
Lets assume that what you see is the interstellar gas glowing. The density of interstellar gas is roughly 1 particle per cm³, or 1E6 particles per m³. Further follow our APOD masters: the glowing object is 25 ly in diameter (I assume a sphere). 1 ly is approximately 1E16 m, so the volume of this sphere is of the order of 1E47m³. That means 1E53 particles. 1E24 particles give one mole, so within the sphere there are 1E29 moles of interstellar gas. Lets follow your assumption: the interstellar gas is made of (neutral) oxygen. 1 mole of neutral oxygen has a mass of 0.01 kg, so the mass of the particles within the sphere is 1E27 kg, Jupiter alike mass. The mass of the sun is 2E30 kg. IMO interstellar gas is mainly neutral hydrogen, emitting 21 cm radio waves, but one order of magnitude less in mass. The mass of the insterstellar stuff within the sphere is then 1E26 kg, a mass similar to Neptune.
Now the (mostly mine) speculations are brought into the arena. Lets assume the shedding is taking place for at least 100 years. It must be more than 25 years, otherwise we could not see all parts of the glowing bubble. A mass of 2E28 kg would have been shedded during a period of 100 years. That is one to two orders of magnitude more than the interstaller gas. A logical conslusion is that due to such a difference in density the shedded gas will "outglow" the interstellar gas.
IMHO neither of your three points is the case, since you could re-assess your assumption about what you see in the image: the interstellar gas or the shedded gas.
Nevertheless your thoughts triggered a philosophical question. On earth, the moon, Venus, Mercury, Mars and the moons of the gas giants Silicon seems to the the abundant element. Si is one of the elements in the nuclear fusion chain of stars. Fe (Iron) is the last one. So there must be somewhat more Si than Fe. Nevertheless the abundancy of Si is overwhelming. Why are our beaches made of silicon oxide and not of grains of rust (iron oxide)?
Regards,
Henk
21 cm: the universal wavelength of hydrogen
Henk
21 cm: the universal wavelength of hydrogen
Onions, chopped and slightly fried
From the wikipedia (http://en.wikipedia.org/wiki/Stellar_evolution)BMAONE23 wrote:Many people have proposed that stars form layers like onions as opposed to a generalized mixing of gasses. Perhaps the Oxygen might be just that particular layer of the star that was previously shed
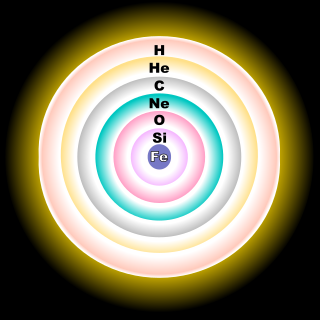
Regards,
Henk
21 cm: the universal wavelength of hydrogen
Henk
21 cm: the universal wavelength of hydrogen
- neufer
- Vacationer at Tralfamadore
- Posts: 18805
- Joined: Mon Jan 21, 2008 1:57 pm
- Location: Alexandria, Virginia
Re: Onions, chopped and slightly fried
----------------------------------------------henk21cm wrote:From the wikipedia (http://en.wikipedia.org/wiki/Stellar_evolution)BMAONE23 wrote:Many people have proposed that stars form layers like onions as opposed to a generalized mixing of gasses. Perhaps the Oxygen might be just that particular layer of the star that was previously shed
<<http://antwrp.gsfc.nasa.gov/apod/ap080117.html
Wolf-Rayets stars are divided into 3 classes based on their spectra, the WN stars (nitrogen dominant, some carbon), WC stars (carbon dominant, no nitrogen), and the rare WO stars with C/O < 1.
The WN stars optical spectra show emission lines from H, NIII (4640Å), NIV, NV, HeI, HeII, and from CIV at 5808Å. In the UV, there are strong emission features from NII, NIII, NIV, NV, CIII, CIV, HeII, OIV, OV, and SiV.
The WC stars optical spectra show emission lines from H, CII, CIII (5696Å), CIV (5805Å), OV (5592Å), HeI, and HeII. No nitrogen lines are seen in the WC stars. In the UV, there are strong emission features from CII, CIII, CIV, OIV, OV, SiIV, HeII, FeIII, FeIV, and FeV.
Wolf-Rayet galaxies are galaxies that contain a large population of Wolf-Rayet stars. These emission-line galaxies show a broad 4686 HeII emission feature due to the WR stars. About one-quarter of these galaxies also show broad NIII 4640 A emission.>>
------------------------------------------
http://antwrp.gsfc.nasa.gov/apod/ap080424.html
http://antwrp.gsfc.nasa.gov/apod/ap060706.html
http://antwrp.gsfc.nasa.gov/apod/ap031016.html
http://antwrp.gsfc.nasa.gov/apod/ap000802.html
http://antwrp.gsfc.nasa.gov/apod/ap080522.html
http://antwrp.gsfc.nasa.gov/apod/ap040421.html
http://antwrp.gsfc.nasa.gov/apod/ap030410.html
http://antwrp.gsfc.nasa.gov/apod/ap970103.html
http://antwrp.gsfc.nasa.gov/apod/ap970102.html
http://antwrp.gsfc.nasa.gov/apod/ap030325.html
http://antwrp.gsfc.nasa.gov/apod/ap990409.html
http://antwrp.gsfc.nasa.gov/apod/ap970928.html
Art Neuendorffer
Re: NGC 6888: The Crescent Nebula - APOD 13 Aug 2008
Density and reactivity. Iron is significantly denser than silicon, and so the majority of the iron in Earth's make-up sank to the core of the planet as it was settling down, while the lighter silicates floated to the top. Also, SiO2 is a very stable molecule, compared to iron oxides which become soluble in acidic conditions.henk21cm wrote:Nevertheless your thoughts triggered a philosophical question. On earth, the moon, Venus, Mercury, Mars and the moons of the gas giants Silicon seems to the the abundant element. Si is one of the elements in the nuclear fusion chain of stars. Fe (Iron) is the last one. So there must be somewhat more Si than Fe. Nevertheless the abundancy of Si is overwhelming. Why are our beaches made of silicon oxide and not of grains of rust (iron oxide)?
Don't just stand there, get that other dog!
- Chris Peterson
- Abominable Snowman
- Posts: 18594
- Joined: Wed Jan 31, 2007 11:13 pm
- Location: Guffey, Colorado, USA
- Contact:
Re: NGC 6888: The Crescent Nebula - APOD 13 Aug 2008
Iron is dense, and sinks to the center of large objects while they are still molten. This is certainly true for all of the terrestrial bodies you list above. It is the outer portions of large bodies that is rich in Si, a light element. And in the case of Mars, there is still enough iron oxide on its "beaches" to make it the Red Planet.henk21cm wrote:Nevertheless your thoughts triggered a philosophical question. On earth, the moon, Venus, Mercury, Mars and the moons of the gas giants Silicon seems to the the abundant element. Si is one of the elements in the nuclear fusion chain of stars. Fe (Iron) is the last one. So there must be somewhat more Si than Fe. Nevertheless the abundancy of Si is overwhelming. Why are our beaches made of silicon oxide and not of grains of rust (iron oxide)?
Chris
*****************************************
Chris L Peterson
Cloudbait Observatory
https://www.cloudbait.com
*****************************************
Chris L Peterson
Cloudbait Observatory
https://www.cloudbait.com
Re: NGC 6888: The Crescent Nebula - APOD 13 Aug 2008
Altough it is Chris' quote, Qev's answer is similar. Basically it is the well known fact that an object with a volumetric mass larger than that of water does not float on water. I was aware of that idea, however it does not fully account for the natural occurence of iron.Chris Peterson and Qev wrote: Iron is dense, and sinks to the center of large objects while they are still molten. This is certainly true for all of the terrestrial bodies you list above. It is the outer portions of large bodies that is rich in Si, a light element. And in the case of Mars, there is still enough iron oxide on its "beaches" to make it the Red Planet.
The ρ of iron is nearly 8000 kg/m³, the ρ of silicon is nearly 2500 kg/m³. Since a lot of time has gone since the earth was liquid, there was an ample amount of time for iron to sink to the center of the earth, whereas silicon would rise to the outer range of the earth. As a result iron would be a rare commodity. To find iron or iron ore, one should have to dig rather deep, at least thousands of kilometers. Since volcanoes are outlets of the inner earth, the material spewn out has an origin usually not very deep, of the order of 10 km. That can not account for the not so sporadic availability of iron.
Tha availability of iron does not comply with the extreme sporadic occurence as descripted above.
Regards,
Henk
21 cm: the universal wavelength of hydrogen
Henk
21 cm: the universal wavelength of hydrogen
- Chris Peterson
- Abominable Snowman
- Posts: 18594
- Joined: Wed Jan 31, 2007 11:13 pm
- Location: Guffey, Colorado, USA
- Contact:
Re: NGC 6888: The Crescent Nebula - APOD 13 Aug 2008
Surface iron is rare, in comparison to the core, which is where nearly all of it ended up. The key point- which you make- is that we find iron ore. We don't find veins of metallic iron (they do exist, but are extremely rare). We find iron bound up with other elements, in complex minerals.henk21cm wrote:The ρ of iron is nearly 8000 kg/m³, the ρ of silicon is nearly 2500 kg/m³. Since a lot of time has gone since the earth was liquid, there was an ample amount of time for iron to sink to the center of the earth, whereas silicon would rise to the outer range of the earth. As a result iron would be a rare commodity. To find iron or iron ore, one should have to dig rather deep, at least thousands of kilometers. Since volcanoes are outlets of the inner earth, the material spewn out has an origin usually not very deep, of the order of 10 km. That can not account for the not so sporadic availability of iron.
The Earth didn't end up as an onion with neat shells of decreasing density for many reasons: it cooled fairly quickly, there was mixing- turbulent and not, elements reacted to form compounds, local cooling and crystallization created traps that stopped differentiation.
Chris
*****************************************
Chris L Peterson
Cloudbait Observatory
https://www.cloudbait.com
*****************************************
Chris L Peterson
Cloudbait Observatory
https://www.cloudbait.com
Re: Onions, chopped and slightly fried
As stated in the Wikipedia entry, this illustration is only for the core of a massive star having at least 8 solar masses. For a less massive star, like the Sun, the fusion reaction would stop at the carbon stage, resulting in a carbon-oxygen white dwarf.henk21cm wrote:From the wikipedia (http://en.wikipedia.org/wiki/Stellar_evolution)BMAONE23 wrote:Many people have proposed that stars form layers like onions as opposed to a generalized mixing of gasses. Perhaps the Oxygen might be just that particular layer of the star that was previously shed
Gary
Fight ignorance!
Re: NGC 6888: The Crescent Nebula - APOD 13 Aug 2008
I would have thought that since iron is the very last fusion product in a massive star's core, it would all be squeezed into neutrons (proton + electron -> neutron + neutrino) during the core collapse, resulting in either a neutron star or, if the core collapse doesn't stop there, a black hole. So, none of the iron would have escaped from the core in a supernova blast.henk21cm wrote: Nevertheless your thoughts triggered a philosophical question. On earth, the moon, Venus, Mercury, Mars and the moons of the gas giants Silicon seems to the the abundant element. Si is one of the elements in the nuclear fusion chain of stars. Fe (Iron) is the last one. So there must be somewhat more Si than Fe. Nevertheless the abundancy of Si is overwhelming. Why are our beaches made of silicon oxide and not of grains of rust (iron oxide)?
Most likely source of iron in interstellar space would be from the shock wave and the intense neutrino flux slamming into the infalling outer shells and being absorbed by the nuclei of the infalling matter, thus creating radioactive isotopes of heavy elements, including iron. The brightness spike of a supernova comes from the rapid fissioning of the unstable isotopes plus the heat of the collision.
Gary
Fight ignorance!
Re: NGC 6888: The Crescent Nebula - APOD 13 Aug 2008
At elementary school the Kiruna mines in Sweden were taught to contain rather pure iron. I do remember about 70%, Google learned me that during nearly 50 years my memory slipped a little, the ore found it is just 60% pure. Later during chemistry class we learned the process to create iron from ore: use cokes and hydrogen gas to bind the oxygen to the hydrogen. Browsing the handbook of chemistry and physics i foud that iron oxyde melts at about 1500 C, silicium oxyde at about 1700 C. So there is not much difference between the two melting points. The density of iron oxide is about 5500 kg/m³, the density of silicium oxide is 2200 kg/m³. Iron oxide will sink just like pure iron to the core, whereas the silicium oxide will float on top.Chris Peterson wrote: Surface iron is rare, in comparison to the core, which is where nearly all of it ended up. The key point- which you make- is that we find iron ore. We don't find veins of metallic iron (they do exist, but are extremely rare). We find iron bound up with other elements, in complex minerals.
The Earth didn't end up as an onion with neat shells of decreasing density for many reasons: it cooled fairly quickly, there was mixing- turbulent and not, elements reacted to form compounds, local cooling and crystallization created traps that stopped differentiation.
Between the earth' crust and the inner core massive convection zones exist. The movement hot material is responsible for the earth' magnetic field. (It can't be any ferromagnetic effect of the iron core, since its temperature is far above the Curie temperature, of about 1000 K.) These convection zones might be responsible for some mixing of silicium- and iron ores. If this concept is true, the consequence is that good quality iron ore could be found on Iceland and e.g. Hawai, where a lot of vulcanoes are active.
starnut wrote:As stated in the Wikipedia entry, this illustration is only for the core of a massive star having at least 8 solar masses. For a less massive star, like the Sun, the fusion reaction would stop at the carbon stage, resulting in a carbon-oxygen white dwarf.
The onion was brought into the discussion since BMAONE23 referred to a layered model of a star. Popular stellar models during the sixties used such an analogon as well. You are right that an iron sun is not likely.
Regards,
Henk
21 cm: the universal wavelength of hydrogen
Henk
21 cm: the universal wavelength of hydrogen
- Chris Peterson
- Abominable Snowman
- Posts: 18594
- Joined: Wed Jan 31, 2007 11:13 pm
- Location: Guffey, Colorado, USA
- Contact:
Re: NGC 6888: The Crescent Nebula - APOD 13 Aug 2008
Be cautious how you interpret melting points. These are normally given for standard atmospheric pressure. At the extreme pressures found deep in the Earth, material properties are very different, and not always well understood. Even at very high temperatures, materials are extremely viscous. The rate at which a denser material will fall through a less dense one is extremely slow, and convective effects may dominate.henk21cm wrote:Browsing the handbook of chemistry and physics i foud that iron oxyde melts at about 1500 C, silicium oxyde at about 1700 C. So there is not much difference between the two melting points. The density of iron oxide is about 5500 kg/m³, the density of silicium oxide is 2200 kg/m³. Iron oxide will sink just like pure iron to the core, whereas the silicium oxide will float on top.
Certainly, convective processes in the mantle are responsible in part for such mixing. They have nothing to do with the Earth's magnetic field, however. That is produced by electric currents in the outer (liquid) core, which is mostly iron, and doesn't interact strongly with the overlying mantle.Between the earth' crust and the inner core massive convection zones exist. The movement hot material is responsible for the earth' magnetic field. (It can't be any ferromagnetic effect of the iron core, since its temperature is far above the Curie temperature, of about 1000 K.) These convection zones might be responsible for some mixing of silicium- and iron ores.
Chris
*****************************************
Chris L Peterson
Cloudbait Observatory
https://www.cloudbait.com
*****************************************
Chris L Peterson
Cloudbait Observatory
https://www.cloudbait.com