JohnD wrote: ↑Sun Jun 11, 2023 9:00 am
Ann,
I understand about absorbtion of colours, but am puzzled. For instance, sodium vapour street lights give off the same wavelength of light that identifies sodium in the Sun. But the sodium in the Sun must be at much higher temperature than in the street light, so why does the solar sodium not glow and fill the dark patch in the spectrum?
Thanks for an explanation!
John
It's easier for me to talk about hydrogen lines and hydrogen emission than to talk about sodium lines, so I'll start with hydrogen. But you must understand that a lot of this has to do with math, and there I'm on very shaky ground!
Take a look at the Trifid Nebula:
The Trifid Nebula, the pink as well as the blue part of it, was born from a cloud of cold, neutral gas, mostly made of hydrogen. This cloud was mostly invisible, although trace amounts of heavier elements like carbon, silicates and ices may have made it look dark. The cloud contracted and gave birth to a cluster of stars, most notably HD 164492, a star of spectral class O7.5V. This hot star ionized the hydrogen in the part of the Trifid Nebula that is pink.
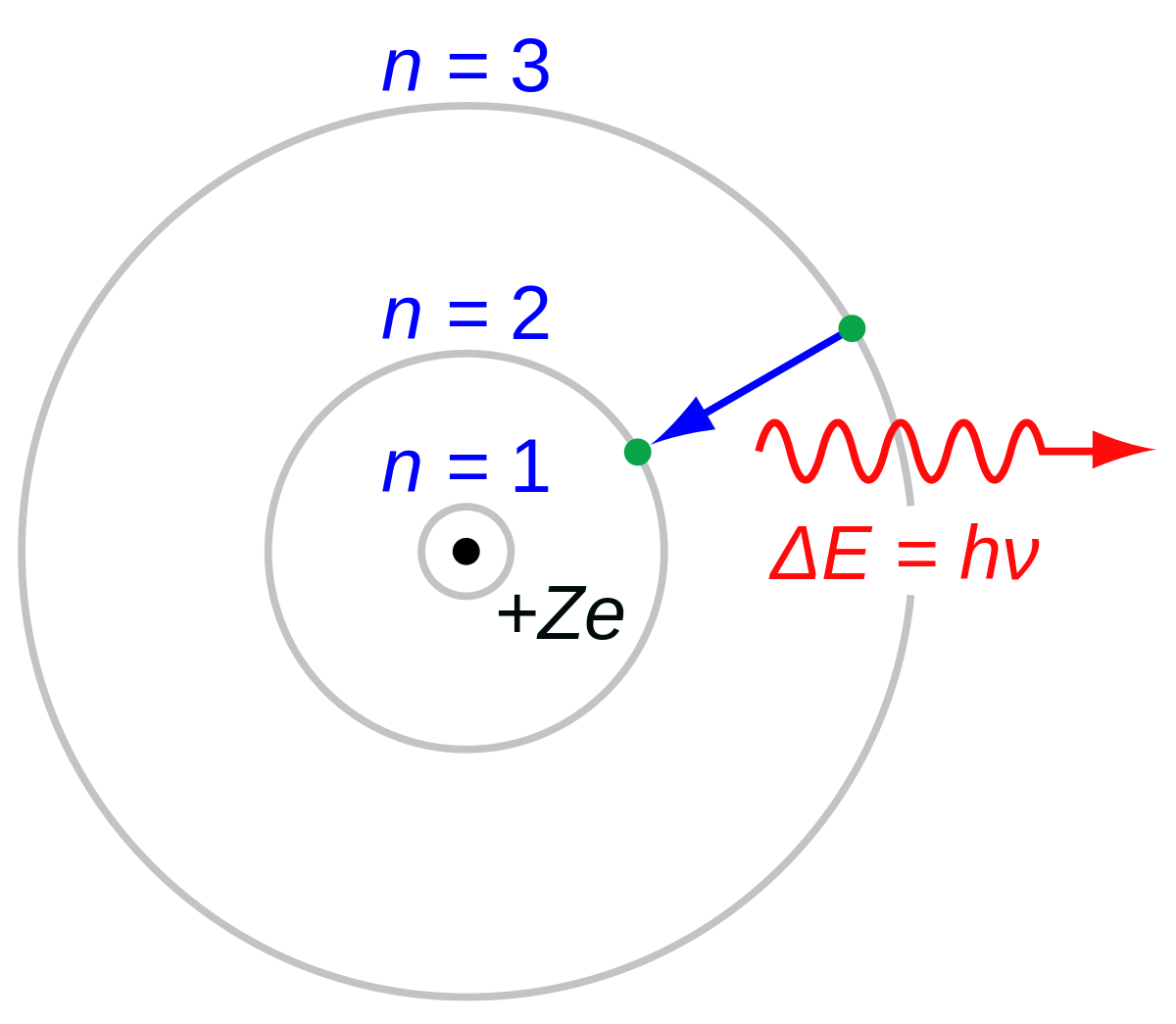
This is what happens after a hydrogen atom has become ionized:
You can see the core of the hydrogen atom, the proton. There is a single electron, which will normally be located in the electron shell designated
N = 2. (Don't ask me about the electron shell designated
N = 1, and
please don't ask me what it means, in quantum mechanics terms, what it means that an electron is "located" somewhere.)
Anyway. When an energetic photon from an O-type star hits a hydrogen atom, the electron will gain so much energy from the photon that hit it that it can "jump up" to the next level, that is, to the shell designated
N = 3. Now the hydrogen atom has become ionized.
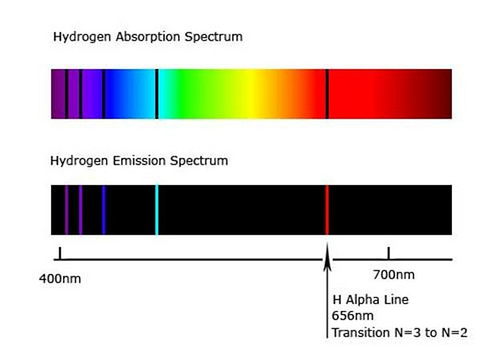
But the electron will not stay in the higher electron shell. It will fall down again, and as it does so, it will release a photon whose exact wavelength is 656.279 nm. This is hydrogen alpha. The color of hydrogen alpha is deep red,
███. But in an emission nebula, the hydrogen alpha will almost always be mixed with some hydrogen beta light. Hydrogen beta is created when an electron is hit by such an energetic photon that the electron "jumps up two levels", and when it falls down, it releases a photon of 486.135 nm. This is a bluish cyan color,
███, and when hydrogen alpha is mixed with some hydrogen beta, the result is the pink color typical of emission nebulas. Maybe this color,
███, but I'm really just guessing.
So much for the emission of hydrogen alpha. The way I understand it, the ionization of sodium should work in a similar manner. A neutral sodium atom should become ionized so that one (or more?) electrons are made to "jump up" to a higher electron shell. When the electron falls down again, it emits yellow sodium light.
Usp.br wrote:
The sodium spectrum is dominated by the bright doublet known as the Sodium D-lines at 588.9950 and 589.5924 nanometers. From the energy level
diagram it can be seen that these lines are emitted in a transition from the 3p to the 3s levels. The line at 589.0 has twice the intensity of the line at 589.6 nm.
I'm sure that made you so much wiser! In any case, sodium light would be this color,
███.
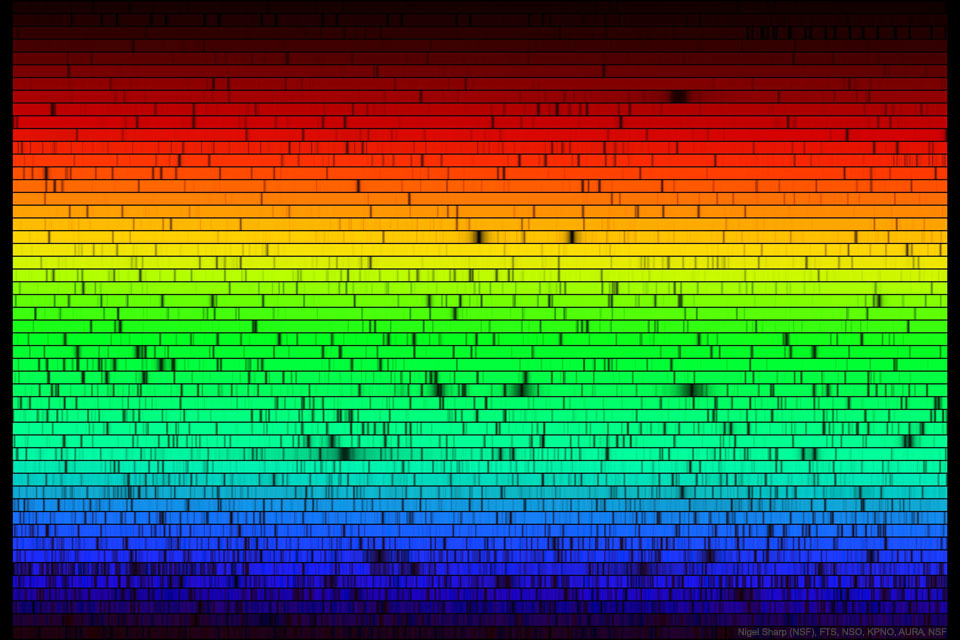
So much for hydrogen or sodium emission of light: It would happen when an electron in a neutral hydrogen or sodium atom is hit by an energetic photon and is "kicked upstairs" to another electron shell. The specific hydrogen alpha or singly ionized sodium light would be emitted when the electron "falls back" again.
I understand the emission mechanism,
more or less. I'm so much more vague when it comes to the absorption of wavelengths inside the Sun. But I do know that the same elements and the same wavelengths are involved:
Khan Academy wrote:
Emission lines refer to the fact that glowing hot gas emits lines of light, whereas absorption lines refer to the tendency of cool atmospheric gas to absorb the same lines of light.
In emission nebulas, cool neutral gas is heated up and ionized by energetic photons. But inside the Sun, almost all the gas is already ionized, except near the surface where the temperature is too low. After all, the temperature at the Sun's core is some 15 million K, but at the photosphere it is less than 6,000 K. If you ask my poor brain to guess, I'd guess that elements that are ionized near the Sun's core become neutral near the Sun's outermost layers. I guess that photons inside the Sun of specific wavelengths, such as 656 nm or 589 nm, get absorbed by relatively (relatively!) cool hydrogen or relatively cool sodium near the Sun's "edge" or photosphere.
During a solar eclipse, you can sometimes see hydrogen alpha along the solar limb.
All right, Johnny, that's the best I can do for you!
Ann
 The Sun and Its Missing Colors
The Sun and Its Missing Colors