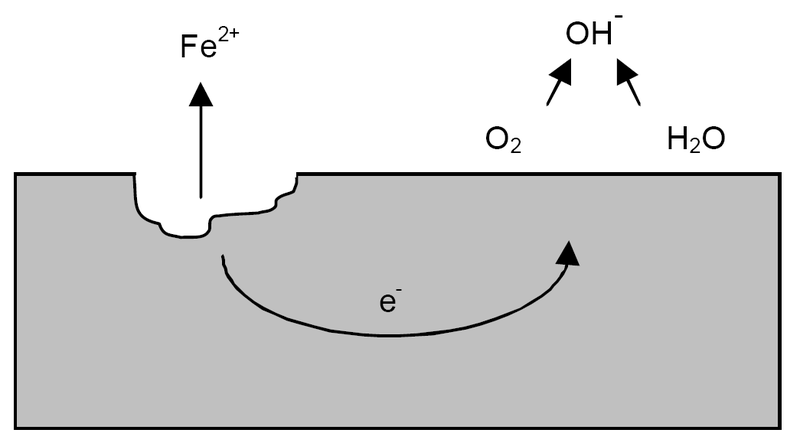
http://en.wikipedia.org/wiki/Pitting_corrosion wrote:
<<Pitting corrosion, or pitting, is a form of extremely localized corrosion that leads to the creation of small holes in the metal. The driving power for pitting corrosion is the depassivation of a small area, which becomes anodic while an unknown but potentially vast area becomes cathodic, leading to very localized galvanic corrosion. The corrosion penetrates the mass of the metal, with limited diffusion of ions. The mechanism of pitting corrosion is probably the same as crevice corrosion.
This kind of corrosion is extremely insidious, as it causes little loss of material with small effect on its surface, while it damages the deep structures of the metal. The pits on the surface are often obscured by corrosion products. Pitting can be initiated by a small surface defect, being a scratch or a local change in composition, or a damage to protective coating. Polished surfaces display higher resistance to pitting.
Alloys most susceptible to pitting corrosion are usually the ones where corrosion resistance is caused by a passivation layer: stainless steels, nickel alloys, aluminum alloys. Metals that are susceptible to uniform corrosion in turn do not tend to suffer from pitting. Thus, a regular carbon steel will corrode uniformly in sea water, while stainless steel will pit.
The presence of
chlorides, e.g. in sea water, significantly aggravates the conditions for formation and growth of the pits through an autocatalytic process. The pits become loaded with positive metal ions through anodic dissociation. The Cl− ions become concentrated in the pits for charge neutrality and encourage the reaction of positive metal ions with water to form a hydroxide corrosion product and H+ ions. Now, the pits are weakly acidic, which accelerates the process.
Besides chlorides, other anions implicated in pitting include thiosulfates (S
2O
32−), fluorides and iodides. Stagnant water conditions favour pitting. Thiosulfates are particularly aggressive species and are formed by partial oxidation of pyrite, or partial reduction of sulfate. Thiosulfates are a concern for corrosion in many industries: sulfide ores processing, oil wells and pipelines transporting soured oils, Kraft paper production plants, photographic industry, methionine and lysine factories.
A single pit in a critical point can cause a great deal of damage. One example is the explosion in Guadalajara, Mexico on April 22, 1992, when gasoline fumes accumulated in sewers destroyed kilometers of streets. The vapors originated from a leak of gasoline through a single hole formed by corrosion between a steel gasoline pipe and a zinc-plated water pipe.>>
 Unusual Hollows Discovered on Planet Mercury
Unusual Hollows Discovered on Planet Mercury